The Lund University Transgenic Core Facility
The Lund University Transgenic Core Facility (LUTCF) is a core resource whose primary goal is to deliver most up-to-date methodologies in transgenic technology to both our researchers and external users.
Services
Established in response to a need for in-house mouse services, the LUTCF provides expertise in cryopreservation of embryos by IVF or natural matings, sperm cryopreservation, rederivation services, strain expansion by IVF, ES morula/blastocyst injections, pronuclear DNA microinjections, and injection of CRISPR edited DNA. These are described in more detail below.
Morula/Blastocyst injections
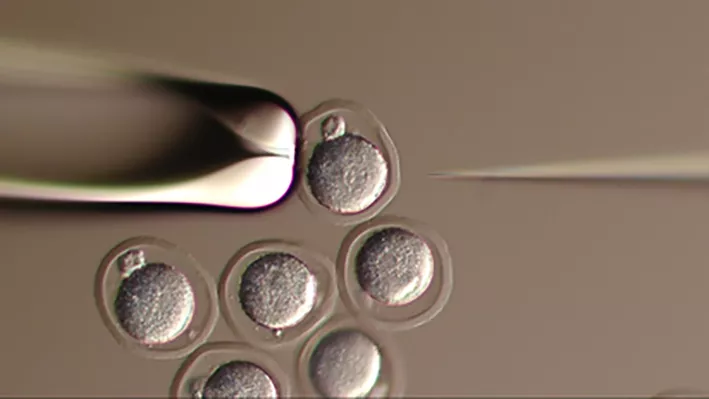
This is currently the most popular method for the generation of targeted knock-out and knock-in models by ES-cell injections into 8-cell morulae and/or blastocysts of mouse embryos.
Quality of the ES cells is of paramount importance in the success of this procedure. Good quality ES cells of low passage are recommended. There are no in-vitro tests to identify clone quality. Two attempts are recommended per construct. Although good quality ES cells can provide chimeras, we do not guarantee germline transmission.
ES-cell expansion of the line can be requested for an additional fee if the requester is off-site.
We guarantee the injection of 50 morulae/blastocysts per clone and transfers into 3.5 dpc (days post-coitum) females. If requested, we can breed chimeras to test for germline transmission. Studs and donor females are charged to the Investigators so any strain can be requested. The standard strains are CD-1, C57BL/6N (or J) depending on the background of the ES-cell line. The opposite coat color of the host strain is needed to identify the resulting chimeras and contribution of ES-cells by coat-color.
Pronuclear injections/CRISPR
We offer pronuclear injections for the creation of non-targeted genetic mutations or the generation of knock-in and knock-out mutations by CRISPR methodology.
Used to produce transgenic mice, pronuclear microinjection involves the injection of a DNA transgene into the nucleus of recently fertilized 1-cell embryos. Random incorporation into the genome results in founder mice that may be mosaic, depending on the stage when the insertion occurs. Once bred into a line, the transgene is present in every cell of the resulting mouse.
Transgenesis is highly dependent on the quality and correct quantification of DNA. Investigators are expected to provide supporting evidence for the quality of the DNA to be injected. DNA purification and CRISPR DNA quality assurance must be provided by the Investigator.
Studs and donor females are charged to the Investigators so that any strain can be requested for pronuclear DNA injection. The standard strain for injection of a CRISPR construct is C57BL/6N. For both techniques, we gurantee 100 injected embryos transferred into pseudopregnant recipient females. Two microinjection attempts are usually required per construct.
Cryopreservation
Cryopreservation of embryos and sperm substantially reduces operating costs of lines that Investigators are not willing to sacrifice, that are not in current use, or as a backup of important strains. It offers Investigators the choice of closing projects and starting news ones.
Cryopreservation insures against loss due to genetic drift, reproductive failure, and natural catastrophes. It permits the quick recovery from infections and it also serves as an alternative to shipping live animals which is costly. Most institutions have the internal requirement of having to rederive regardless.
Sperm cryopreservation: Efficiencies are very strain dependent and great variations can be seen even between males of the same lines. The disadvantage is that only one half of a strains genotype is cryopreserved. It is, nonetheless, the recommended method in compliance with the current regulations. It is cheaper, faster and uses far fewer animals on a per line basis. Quality control experiments are conducted on each line to ensure the generation of live pups. Tissue samples are returned to Investigators to re-check genotypes.
Embryo Cryopreservation by IVF is available to investigators who require the background to be maintained, have complicated genetic mutations or as a back-up if the quality control of the sperm cryopreservation attempts do not work well.
a) If a sperm cryopreservation request does not QC adequately, we recommend generating embryos by IVF to supplement the archive. Donor females are ordered for the procedure which can produce very large numbers. Most often, one attempt is all that is required.
b) IVF can also be conducted with Investigators providing both males and females. Plugging rates of males is often a limitation to good embryo yields. IVF circumvents this problem. A stricter serology (SPF) is required as the animals need to be housed in a separate facility.
Embryo Cryopreservation by natural mating: The slowest of the cryopreservation methods, it can be potentially costly as many attempts may be required to reach the goal of increasing the success of the recovery process. Some strains may not breed well and superovulate poorly.
We conduct cryopreservation by vitrification. As a quality control, one embryo straw is thawed and transferred to a recipient mother to generate pups. Each Institution has its own guidelines, but the recommended archive is approximately 250 homozygous cryopreserved embryos or 300-500 heterozygous embryos.
Rederivation
Embryo-derivation refers to the transfer of embryos from donor mice to surrogate mothers housed in pathogen-free rooms. All mouse lines from non-commercial sources must be embryo-derived into Lund BMSD and its affiliated facilities to maintain the specific-pathogen free status by FELASA recommendation. Acceptance of animals is accessed by the veterinarians on a per-case basis.
a) The LUTCF regularly ships and receive fresh and frozen sperm and embryos from other institutions and repositories for their establishment in Lund facilities which include the BMC Barrier and Conventional, MV, CRC Barrier and Conventional and the new Comparative Medicine Unit (expected to be operational in May 2022). The Comparative Medicine Unit will have the highest SPF status in the Biomedical Services Division of Lund. All rederivations will be conducted with the expectation of Investigators retaining a small breeding colony there and shipping experimental animals to affiliated facilities.
b) We conduct rederivations by IVF from fresh sperm samples taken from mice temporarily housed in our Quarantine Facility or the Transgenic Facility lab if the serology is approved by our veterinarians.
c) Because the IVF technique often produces high embryo yields, Strain Expansion is an option for an additional cost with up to 10 recipient mothers transferred with embryos to generate a large number of experimental animals of the same age.
More information
For additional information or information about the costs, please contact transgen [at] med [dot] lu [dot] se