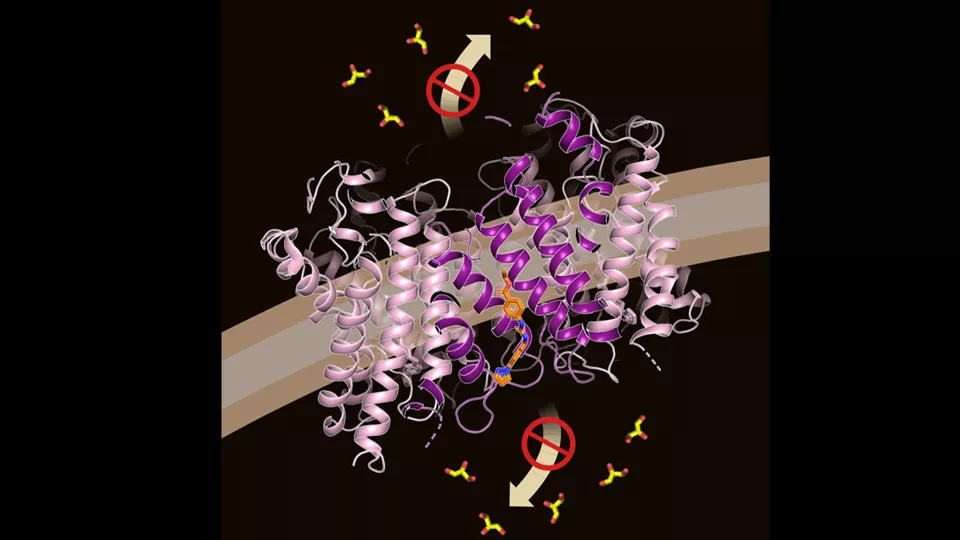
Aquaporins are water channels found in the cell membranes. Their role is to facilitate the transport of water and other smaller molecules to and from cells. In this way, they regulate the flow and pressure within the cell. Aquaporins are central to many mechanisms in our cells, and also play a crucial role in several health conditions such as cancer and type 2 diabetes.
– It seems that cancer cells may increase the number of aquaporins to enhance their own survival and increase their spreading ability, says Karin Lindkvist, professor of cell biology at Lund University and structural biology researcher.
Therefore, it’s interesting to try to develop new drugs that block the activity of aquaporins. But to create new drug candidates, researchers must first gain knowledge of how they bind to aquaporins. Using cryo-electron microscopy, a technology with extremely high resolution, researchers have now, for the first time, managed to produce an image of an aquaporin with a drug candidate bound to the protein. They have also shown that the drug affects the growth capability of leukemia cells by inactivating aquaporins.
– By understanding the structure of the protein, and how drugs attach to it, we are trying to comprehend how cancer cells respond to new drug candidates. This means that we can now begin to develop new and better drug candidates that selectively target certain groups of aquaporins and then proceed to investigate the drug's effectiveness for different types of cancer, concludes Karin Lindkvist.